Table of Contents
Dr. Alberto Sola is one of the world’s leading experts in medically-based ibogaine treatment; he has more clinical experience with safe and effective ibogaine administration than any other M.D. in the world today.

Lambarene
The initial pharmacological, pharmacokinetic, chemical and behavioral studies led to the initial introduction of ibogaine in France during the 1930s, in the form of a pharmaceutical medication, introduced under the tradename Lambarene. Lambarene was marketed in France as a mental and physical stimulant, anti-depressant, and for healthy individuals during times of greater than normal physical or mental exertion.
Lambarene contained approximately 5-8mg. of ibogaine, per tablet. Lambarene was prescribed in cases of depression, asthenia, convalescence, and infectious disease. Lambarene was finally removed from the market in 1967 when the sale of ibogaine-containing products was prohibited.
Ibogaine’s Anti-Addictive Properties
The history of utilizing ibogaine to break the cycle of drug-dependence is relatively short. While it is likely that the U.S. government who dosed inmates with ibogaine at the Lexington, KY, federal “narcotics farm” under the auspices of Harris Isbell, M.D — as well as CIBA pharmaceuticals, who were investigating ibogaine as an anti-hypertensive agent — were both aware of ibogaine’s anti-addictive properties; neither entity chose to share this information with the rest of the world. It is unknown what exactly was contained within Dr. Harris Isbell’s data, because all records have been declared lost. The fact that the research took place, has been recorded; the actual data, purportedly no longer exists. CIBA abandoned further research, because they were unconvinced of ibogaine’s commercial viability.
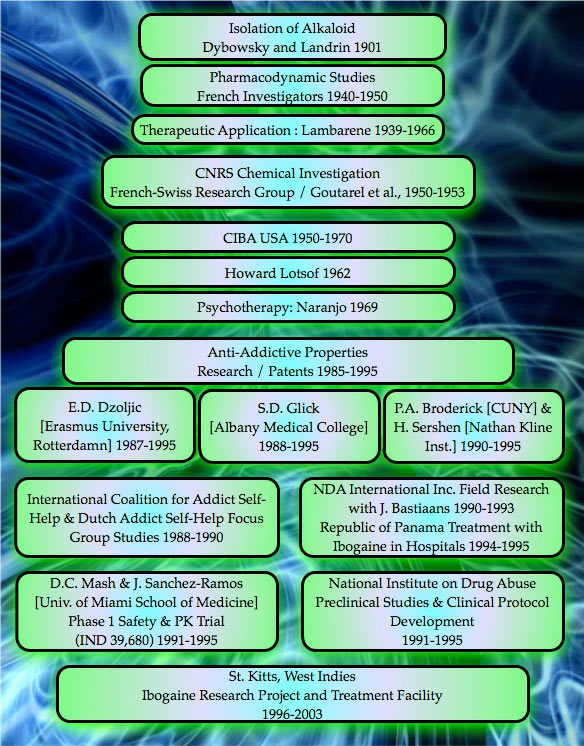
Timeline of Ibogaine Development
Based on anecdotal reports from groups of self-treating drug-dependent individuals in America and Europe, which indicated that a single dose of ibogaine completely obviated opiate withdrawal and produced significant reduction in cravings for opiates, opioids, cocaine, alcohol and other addictive drugs; in 1985 the USPTO (United States Patent and Trademark Office) granted Howard Lotsof a patent for using ibogaine as an ultra-rapid opioid detox.
The USPTO green-lighted Howard’s original patent for ibogaine vs. opiates and semi-synthetics that are derived from natural product chemistry, such as heroin, hydromorphone, and oxycodone; as well as fully synthetic opioids such methadone, meperidine, and fentanyl. After the initial ibogaine patent demonstrating efficacy for ultra-rapid opiate and opioid withdrawal was approved, Mr. Lotsof was granted an additional series of patents between 1986-1992 for utilizing the ibogaine molecule as an ultra-rapid method for interrupting or attenuating a large spectrum of poly-drug dependency syndromes.
Initial Series of Ibogaine Patents:
- 1985 – Rapid method for interrupting the narcotic addiction syndrome (USPTO #: 4,499,096)
- 1986 – Ibogaine vs. stimulants (cocaine and amphetamine. USPTO #: 4,587,243)
- 1989 – Ibogaine vs. alcohol (USPTO #: 4,857,523)
- 1991 – Ibogaine vs. nicotine (USPTO #: 5,026,697)
- 1992 – Ibogaine as a Rapid method for interrupting or attenuating poly-drug dependency syndromes (USPTO #: 5,152,994).
Ibogaine Research Within the United States:
- 1991 – the Medications Development Division of NIDA (the National Institute on Drug Abuse), is impressed enough with the existing scientific evidence showing ibogaine’s putative ability to attenuate withdrawal symptoms, in animal testing, to start investigating ibogaine addiction treatment in human subjects.
- 1992 – July 15th, the FDA (Food and Drug Administration) convenes a Drug Abuse Advisory Committee to review their policy on doing research with Schedule I drugs. The advisory committee recommends that Schedule I drugs should be evaluated using the same standards the FDA uses for all other molecules.
- 1993 – The FDA grants Dr. Deborah C. Mash and scientists at the University of Miami School of Medicine, permission to conduct a limited Phase 1 Pharmacokinetic and Safety Trial using ibogaine in human subjects (IND 39,680).
- 1995 – A review committee at NIDA decides to suspend further funding for ibogaine research. After several years of sustained interest, NIDA decides to cease all funding of the Ibogaine Research Project and marks grant submissions with “Not for Further Consideration.” The end. Due to a total lack of any financial support for clinical research, dose-escalation studies within the United States never progressed beyond this date.